Home /
Expert Answers /
Chemistry /
the-watts-bath-for-electrodepositing-nickel-has-undergone-a-number-of-changes-since-the-original-com-pa271
(Solved): The Watts bath for electrodepositing Nickel has undergone a number of changes since the original com ...
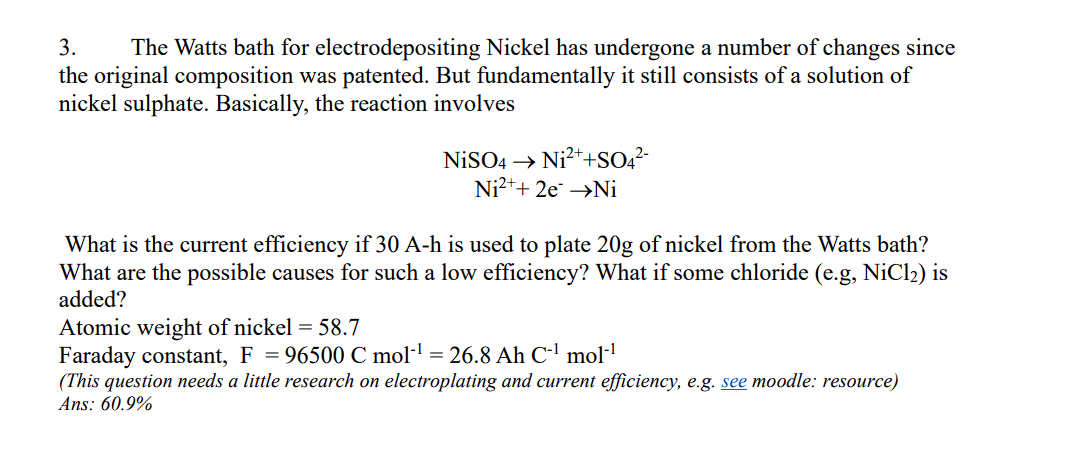
The Watts bath for electrodepositing Nickel has undergone a number of changes since
the original composition was patented. But fundamentally it still consists of a solution of
nickel sulphate. Basically, the reaction involves
NiSO_(4)->Ni^(2+)+SO_(4)^(2-)
Ni^(2+)+2e^(-)->NiNiCl_(2) =58.7
Faraday constant, F=96500Cmol^(-1)=26.8AhC^(-1)mol^(-1)
(This question needs a little research on electroplating and current efficiency, e.g. see moodle: resource)
Ans: 60.9%