Home /
Expert Answers /
Economics /
please-help-nbsp-nbsp-thank-you-2-a-non-conducting-rigid-steel-compartment-is-pa396
(Solved): please help! thank you 2. A non-conducting, rigid steel compartment is ...
please help!
thank you ????????
2. A non-conducting, rigid steel compartment is divided into two non-equal sections by a non- conducting membrane that can move freely and frictionlessly. Initially compartment A contains 5 mol (0.005 kmol) of solid S and 10 mol (0.01 kmol) Nitrogen (N2) at 100 kPa and 298 K. Compartment B contains 0.1 kg wet steam with a quality (xy) of 86%. The steel container is perfectly insulated, but a heating coil in compartment A can deliver heat to its contents. The coil is switched on and heat flows to the contents in compartment A. The slow decomposition of solid S to ideal gases X and Y according to the reaction below: o Ss = X, + Y, with AH, RX 298 15000 mol is allowed to reach equilibrium at a final temperature of 500 K. At this point (equilibrium in compartment A at 500 K), the steam in compartment B is at 125°C How much heat had to flow to the contents of compartment A during the process? (Answer in kJ, round to four significant numbers e.g. 1234, 1.234 or 12.34) n) What is AGRX299 for the decomposition reaction of solid S? Answer in J/mol-round to a whole number
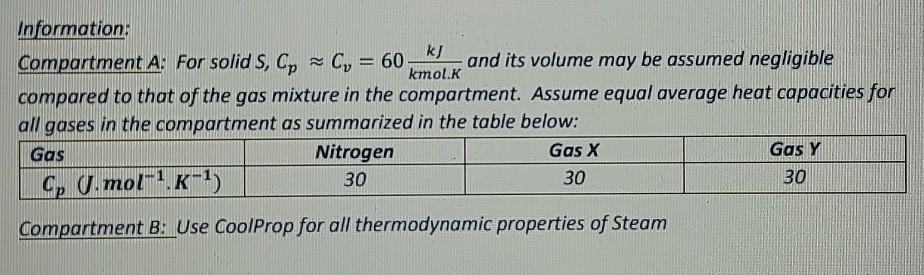
Information: k) Compartment A: For solid S, Cp Cy = 60 and its volume may be assumed negligible kmol.K compared to that of the gas mixture in the compartment. Assume equal average heat capacities for all gases in the compartment as summarized in the table below: Gas Nitrogen Gas X Gas Y Cp U.mol-1K-1) 30 30 Compartment B: Use CoolProp for all thermodynamic properties of Steam 30