Home /
Expert Answers /
Electrical Engineering /
nbsp-a-limestone-calciner-is-used-to-decompose-limestone-caco3-into-quicklime-cao-and-co2-caco-pa613
(Solved): A limestone calciner is used to decompose limestone CaCO3 into quicklime CaO and CO2. CaCO ...
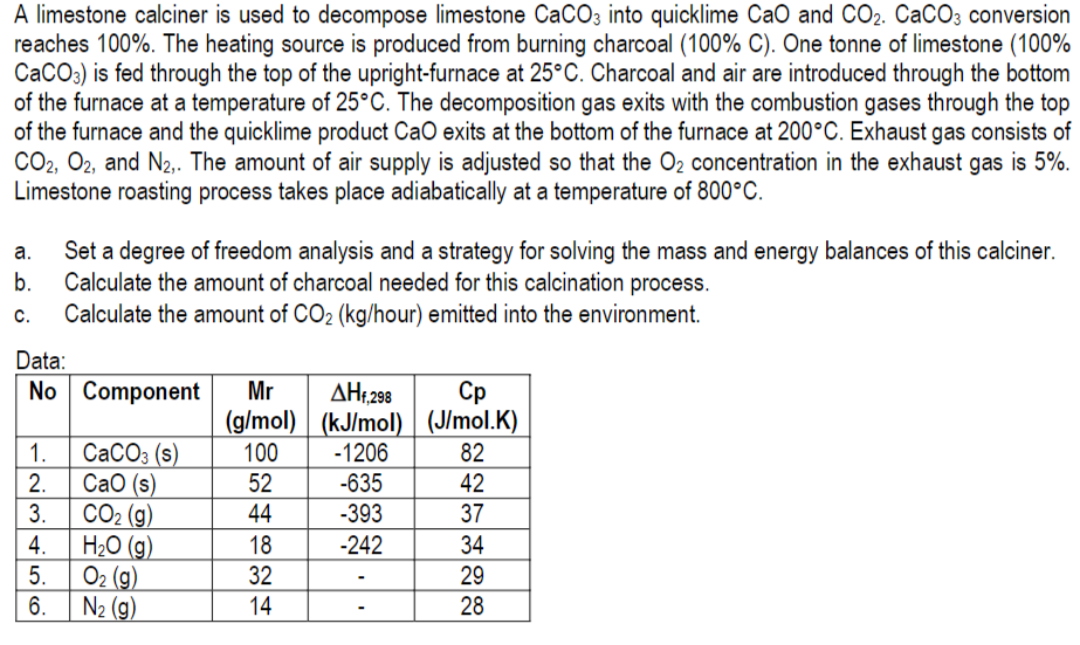
A limestone calciner is used to decompose limestone CaCO3 into quicklime CaO and CO2. CaCO3 conversion reaches 100%. The heating source is produced from burning charcoal (100% C). One tonne of limestone (100% CaCO3) is fed through the top of the upright-furnace at 25°C. Charcoal and air are introduced through the bottom of the furnace at a temperature of 25°C. The decomposition gas exits with the combustion gases through the top of the furnace and the quicklime product CaO exits at the bottom of the furnace at 200°C. Exhaust gas consists of CO2, O2, and N2,. The amount of air supply is adjusted so that the O2 concentration in the exhaust gas is 5%. Limestone roasting process takes place adiabatically at a temperature of 800°C. a. b. Set a degree of freedom analysis and a strategy for solving the mass and energy balances of this calciner. Calculate the amount of charcoal needed for this calcination process. Calculate the amount of CO2 (kg/hour) emitted into the environment. C. Data: No Component OUAWN 1. 2. 3 4. 5 6 CaCO3 (s) CaO (s) CO2 (g) H2O (9) O2 (g) N2 (g) Mr ??, 298 ?? (g/mol) (kJ/mol) (J/mol.K) 100 -1206 82 52 -635 42 44 -393 37 18 -242 34 32 29 14 28
Expert Answer
:: Solution :: Hence,178.57kg of limestone is req